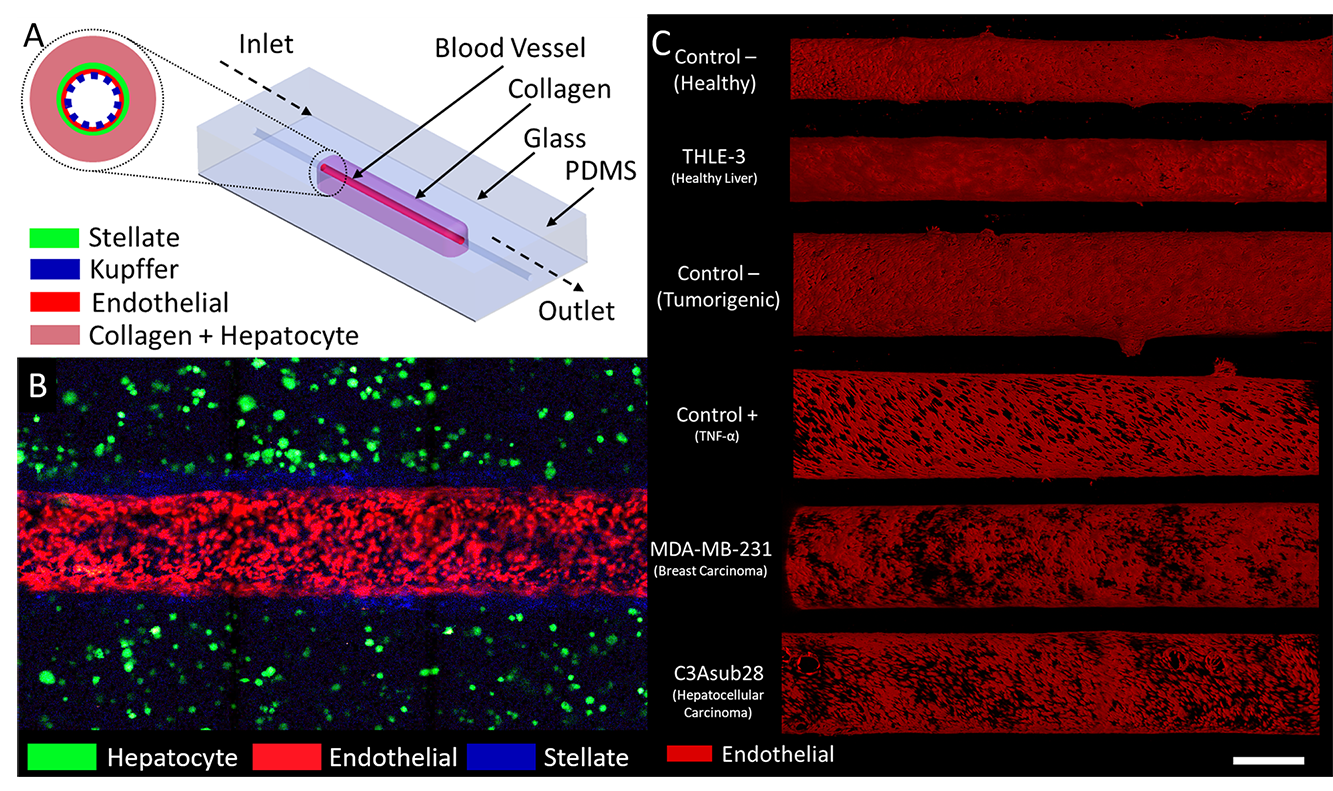
My research is focused on the development of vascularized liver-on-a-chip disease models to predict hepatic response and toxicity associated with hepatocellular carcinoma (HCC), tumor microenvironment or exogenous compounds. My aspiration is to create vascularized platforms that may be extensively utilized for pre-clinical drug screening and observing cellular interactions. The organ-on-chip technology creates an ideal microenvironment that allows the researcher to investigate activities that control human organ functionality at both the molecular and cellular scale, identify new nanoparticles to be conjugated with chemotherapeutic agents and test their effectiveness. This will ensure that adequate drugs of appropriate dose are administered to patients at the right time. The disease models that I’m currently working on include:
Hepatocellular Carcinoma: Having the second lowest survival rate among all cancer types, HCC is commonly diagnosed at an intermediate or advanced stage, which limits the accuracy in prognosis and the availability of treatment options. My goal is to develop a vascularized HCC disease model to investigate cellular interactions and improve the efficiency of chemotherapy treatments.
Chemotherapy Induced Hepatotoxicity: Due to its non-selective nature, chemotherapy can cause significant hepatotoxicity that may result in short term or chronic liver failure. While only a minor portion of the injected chemotherapeutic drug accumulates in the tumor environment, the majority of the drug is deposited in the liver. In addition, the liver-specific metabolic expression can diminish the overall efficacy of drugs. My goal is to create a vascularized healthy liver sinusoid that is connected to breast tumor microenvironments to investigate the interaction, accumulation and transport phenomena of chemotherapeutics, nanoparticles and other compounds.