Meghan Bloom
My research focuses on using quantitative measurements to characterize inflammatory aspects of the tumor microenvironment in order to guide therapeutic strategies. My research investigates pro-inflammatory cell signaling networks, which regulation of are critical in cancer progression. Development of pathway specific therapies have been largely unsuccessful due to the pleiotropic effects targeted drugs have on signaling networks. Specifically, I am doing in vitro studies that perturb the nuclear factor kappa-B (NF-κB) signaling network and quantifying temporal changes in NF-κB activation using immunofluorescent imaging. The goal of this study is to shed light on the importance of pathway analyses when developing targeted drugs to increase therapeutic efficacy. Secondly, I am interested in characterizing inflammatory cell infiltration in preclinical models of HER2+ breast cancer. Inflammation as well as inflammatory-derived changes in the tumor microenvironment can alter a tumor’s sensitivity to treatment. I am currently acquiring longitudinal ex vivo data to quantify changes in tumor inflammation over time and am working to incorporate these data into a mechanistic model to predict optimal time windows for therapy in HER2+ breast cancer.
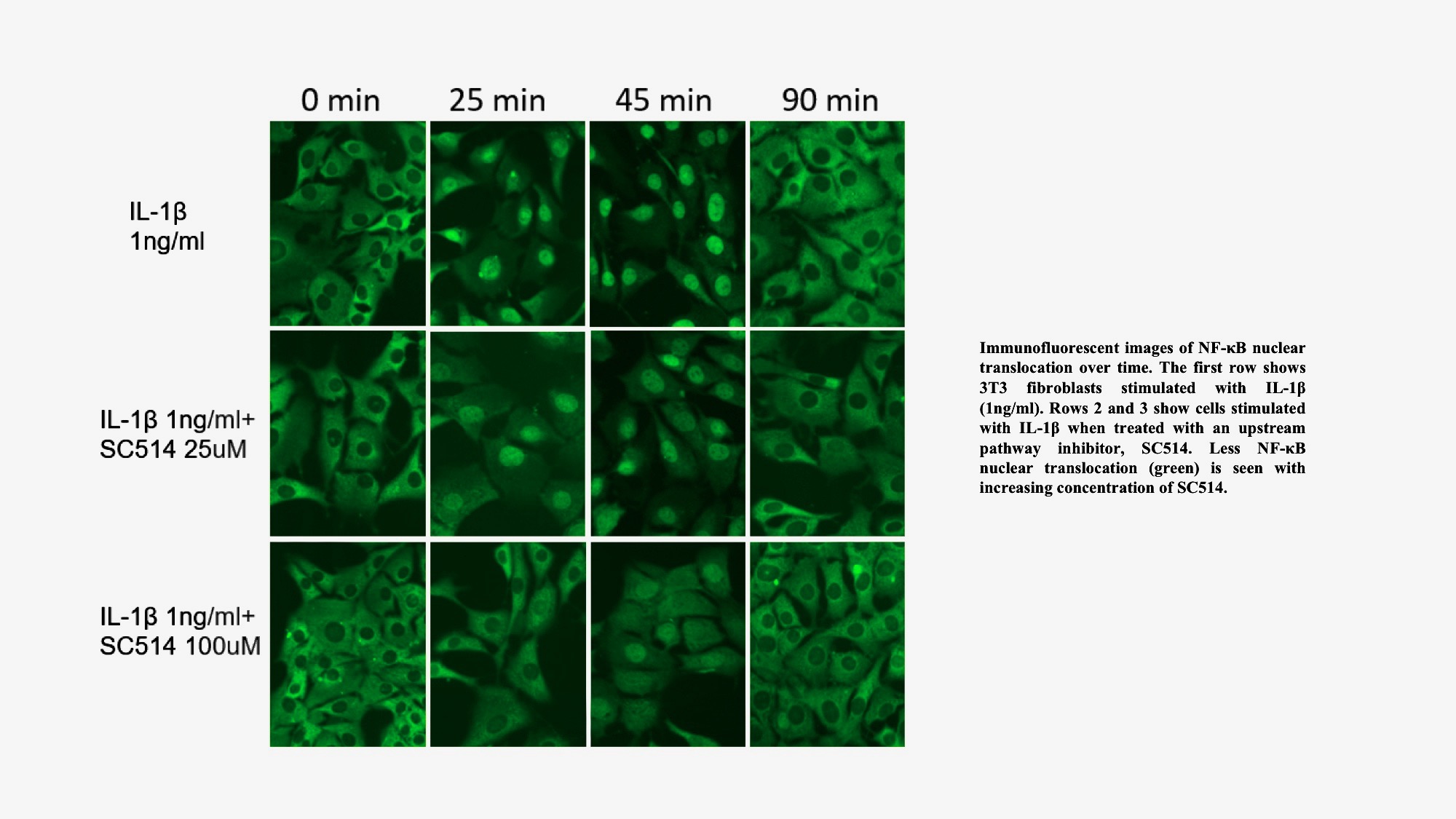
Figure 1: A) Representative images of NF-κB translocation over time. Row 1: untreated 3T3 fibroblast cells, Row 2: cells treated with 25 uM SC-514, Row 3: cells treated with 100 uM of SC-514. All cells were stimulated with IL-1β (1ng/ml) and fixed at 0, 25, 45, and 90 min. NF-κB stained (green) with antibody against NF-κB-p65.